Abstract
Background: SEL, an oral first-in-class inhibitor of XPO1 showed activity in clinical trials for hematologic malignancies. SEL inhibits DNA damage repair and exhibits marked synergy with doxorubicin in preclinical myeloma models. SEL/Dex results in a 20% response rate in penta-refractory myeloma patients. We report here the results of a phase I/II trial of SEL in combination with DOX and Dex in patients with RRMM.
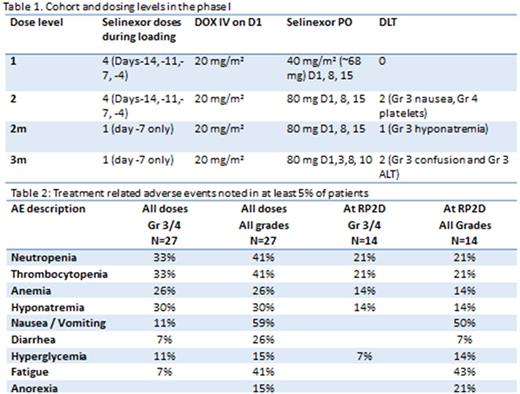
Methods: Eligible patients had RRMM and received ≥ 2 prior therapies including lenalidomide and a proteasome inhibitor. Treatment consisted of a loading phase with SEL and Dex for 1-2 weeks; an induction phase with DOX 20 mg/m2 IV D1, SEL and Dex (once weekly) and a maintenance phase of weekly SEL/Dex. Two loading phases were evaluated: A. SEL/Dex twice weekly for 2 weeks, B. One dose of SEL/Dex (table 1). Primary and secondary end points: maximum tolerated dose (MTD) / recommended phase II dose (RP2D) of the combination, and overall response rate (ORR) per International Myeloma Working Group (IMWG) criteria.
Results: Twenty-seven patients were enrolled (median age of 62 years, median of 6 prior lines (range 2-10)). No dose limiting toxicities (DLT) were noted in dose level (DL) 1. Two patients experienced a DLT in DL2 (Gr4 thrombocytopenia and Gr 3 nausea). The loading phase was shortened to 1 dose of SEL (80mg) on day -7 (DL2m). 1/6 patients experienced a DLT on DL2m (Gr3 hyponatremia). Two patients experienced a DLT in dose level 3m (Grade 3 ALT and Grade 3 confusion). The recommended phase II dose was determined to be DL2m (SEL 80mg D1,8,15, DOX 20mg/m2 D1 and Dex 40 mg D1,8,15). Three patients died on study (1 from RSV pneumonia, one from pneumocystis Jiroveci and 1 from advanced myeloma). The most common Grade 3/4 at least possibly related adverse events are as follows: thrombocytopenia 33%, neutropenia 33%, asymptomatic hyponatremia 30%, anemia 26%, nausea / vomiting 11%, hyperglycemia 11%, diarrhea 7%, and fatigue 7% (table 2). Of the 14 patients treated at the RP2D, one was not evaluable (NE), one had a PR and 1 a MR at the time of this analysis. Per protocol, this current level of response does not justify proceeding with the second stage of the phase II study. Of the 27 total patients treated, we noted 2 VGPR, 2 PR, 3MR, 8SD, 4NE with an ORR of 15%, clinical benefit rate (MR and better) of 26% by intent to treat. There was increased nuclear localization of Ikβ post selinexor treatment in responders compared to non-responders (Nuclear / cytoplasmic ratio mean 1.15 ±0.06 versus 0.93 ±0.09; p=0.04).
Conclusions: The addition of DOX to SEL/Dex, while reasonableywell tolerated, does not appear to improve the ORR noted with SEL/Dex in this heavily pretreated patient population.
Baz: BMS: Research Funding; celgene: Honoraria, Research Funding; karyopharm: Research Funding; takeda: Research Funding; merck: Research Funding; Sanofi: Research Funding. Zonder: Janssen: Consultancy; BMS: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy; Pharmacyclics: Other: Data Safety Monitoring Committee; Prothena: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal